The Ultimate Guide to Autoimmune Management Through Microbiome Modulation
Four Ways Dysbiosis Fuels Autoimmune Disease
Autoimmune diseases are on the rise, and as practitioners, we’re seeing more patients struggling with chronic inflammation, immune dysfunction, and persistent symptoms that don’t always respond to conventional treatments. Emerging research over the past decade has revealed a key player in autoimmunity that we can no longer ignore: the gut microbiome.
Dysbiosis—the imbalance of beneficial and harmful microbes—has been implicated in nearly every major autoimmune condition, from multiple sclerosis (MS) and rheumatoid arthritis (RA) to lupus, type 1 diabetes, and inflammatory bowel disease (IBD) (Cani et al., 2019). But how does the microbiome contribute to autoimmunity? Let’s explore four key mechanisms and how you can use this knowledge in clinical practice.
Take Your Expertise to the Next Level Join the NEW Autoimmune Mentoring Program
Are you ready to expand your knowledge, refine your clinical skills, and integrate the latest microbiome research into your practice?
📢 Join My NEW Brad’s Brainiacs: Autoimmune Mentoring Program!
This mentorship program is designed specifically for practitioners who want to deepen their understanding of autoimmune disease and the microbiome. Whether you're a naturopath, nutritionist, functional medicine doctor, or integrative health professional, this program will provide you with the tools and confidence to implement cutting-edge, evidence-based strategies in your clinic.
🔹 What you’ll gain:
✔️ The latest scientific insights into how the microbiome influences autoimmunity.
✔️ A structured approach to using functional testing like metagenomic sequencing to guide treatment decisions.
✔️ Practical clinical protocols for gut healing, immune modulation, and patient-specific interventions.
✔️ Exclusive case studies and real-world applications to help you apply these insights with confidence.
✔️ Access to a community of like-minded practitioners, sharing experiences, case discussions, and the latest research.
1. Intestinal Permeability and Autoimmunity
When the intestinal barrier is compromised, bacterial toxins and inflammatory metabolites triggers an immune activation. This phenomenon, commonly called "leaky gut," has been linked to autoimmune diseases like lupus, RA, and type 1 diabetes (Mu et al., 2017).
For example, Enterococcus gallinarum, a gut bacterium, has been found in the liver of lupus patients, where it triggers systemic inflammation (Manfredo Vieira et al., 2018). Likewise, children at risk of type 1 diabetes show increased gut permeability before disease onset, suggesting that barrier dysfunction precedes autoimmunity (Vatanen et al., 2018).
Increased gut permeability has also been associated with neuroinflammation in conditions like MS, where tight junction proteins (e.g., occludin, claudin) are often downregulated, allowing pro-inflammatory cytokines and bacterial metabolites to cross the gut-brain axis (Braniste et al., 2014).
Clinical Pearl:
✔️ Consider testing for intestinal permeability markers such as zonulin or Hexa-LPS in patients with suspected autoimmunity.
✔️ Healing the gut barrier with nutrients like glutamine, zinc, and polyphenols (e.g., curcumin, quercetin) may help reduce systemic inflammation.
✔️ Address gut-brain axis dysfunction in neuro-autoimmune cases (e.g., MS, lupus) by modulating gut barrier integrity.
“The gut lining regenerates every 3-5 days! However, chronic stress, NSAIDs, and dysbiosis can slow this repair process, worsening permeability.”
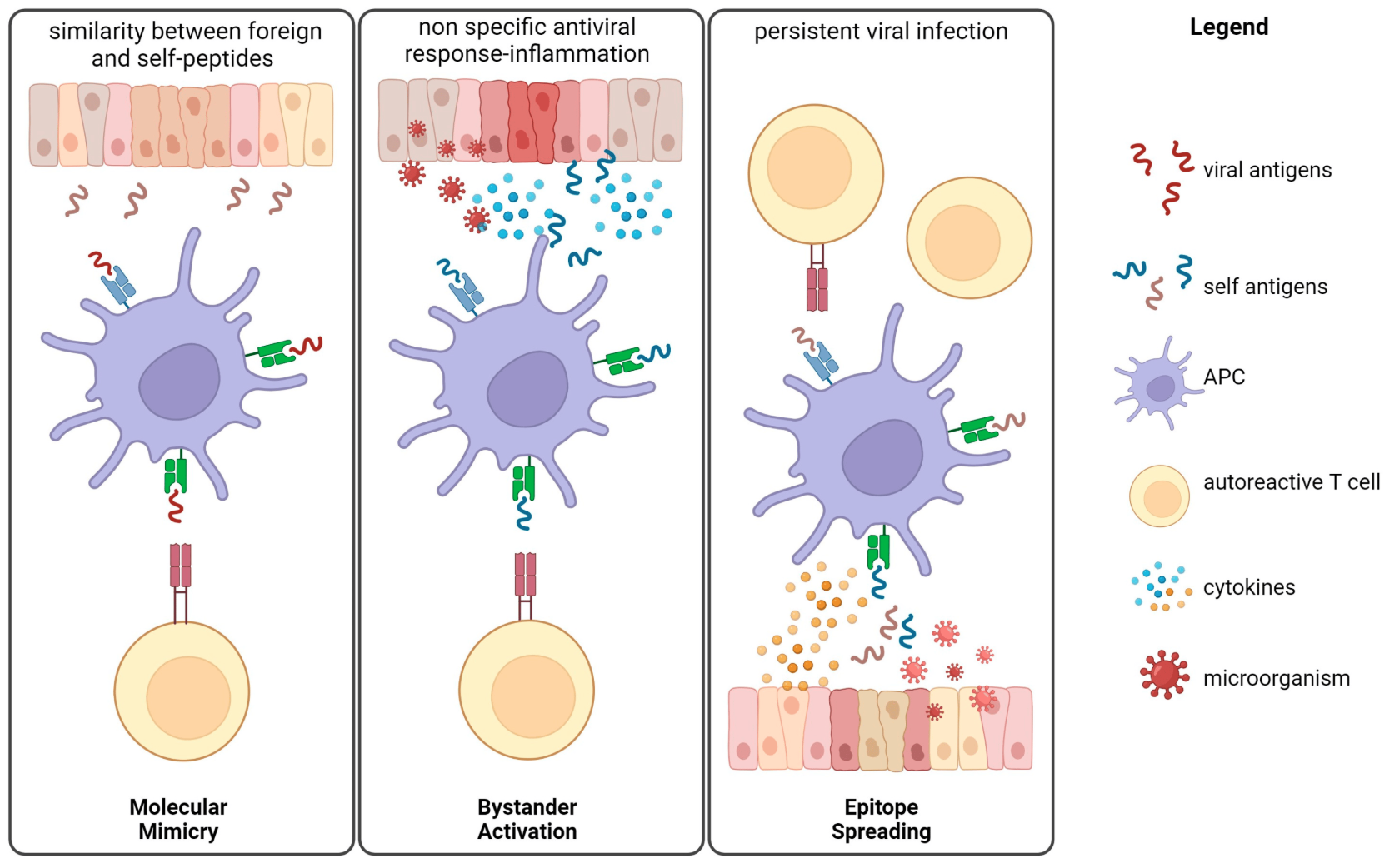
2. Molecular Mimicry and Autoimmunity
Molecular mimicry occurs when microbial proteins resemble self-antigens, causing the immune system to mistakenly attack its own tissues. This is one reason why infections and gut dysbiosis can trigger autoimmunity.
Prevotella copri, commonly elevated in new-onset RA, has peptide sequences that mimic joint proteins, potentially triggering an autoimmune response (Scher et al., 2013).
Klebsiella pneumoniae has been linked to ankylosing spondylitis due to its molecular resemblance to HLA-B27 and collagen (Ebringer & Rashid, 2016).
A recent study found that Clostridium perfringens, a common gut bacterium, produces a surface protein that mimics aquaporin-4, the target of autoantibodies in neuromyelitis optica (NMO). This suggests that gut bacteria may be involved in central nervous system autoimmunity (Wang et al., 2020).
Clinical Pearl:
✔️ Patients with recent infections or significant dysbiosis may benefit from interventions that reduce cross-reactive immune activation, such as probiotics, prebiotic and dietary modifications.
✔️ This does not indicate the need to "kill" these bacteria, but rather feeding up the beneficial species.
✔️ Look for molecular mimicry clues in autoimmune patients with a history of chronic infections or persistent gut issues.
✔️ Assess the oral microbiome in patients with RA and AS—pathogens like Porphyromonas gingivalis can contribute to molecular mimicry and citrullinated autoantigens.
“The HLA-B27 gene, strongly associated with ankylosing spondylitis, is also linked to a heightened immune response to gut bacteria like Klebsiella pneumoniae.”
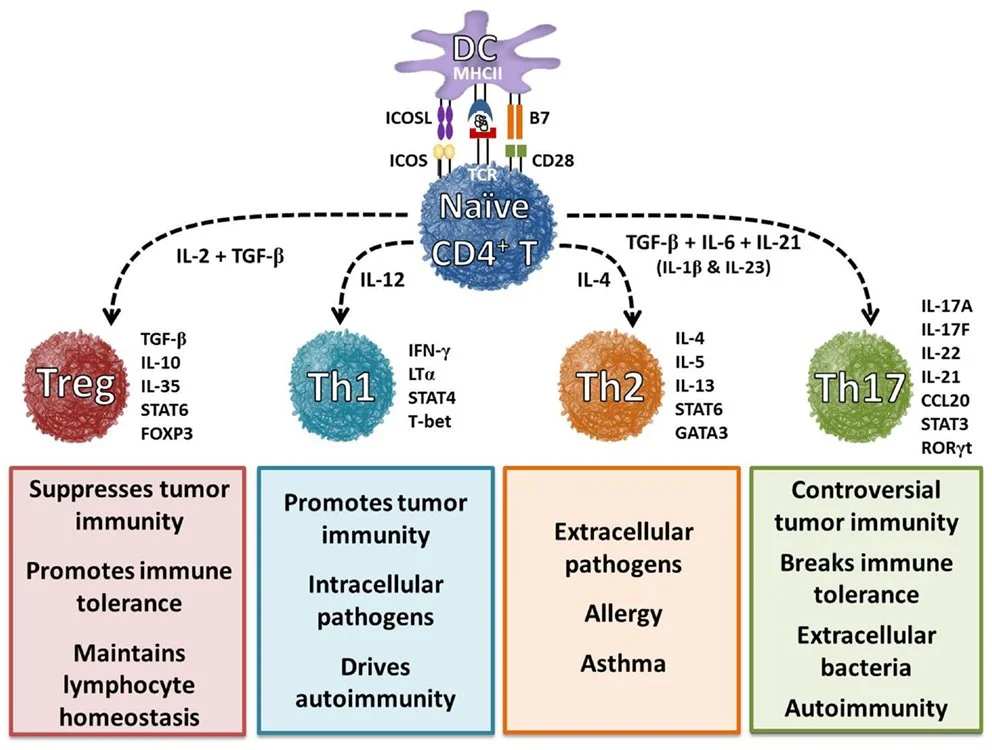
3. TH17 Activation and Autoimmunity
Th17 cells are essential for fighting infections but, when overactive, they contribute to autoimmune conditions like MS, psoriasis, and Crohn’s disease (Lee et al., 2020).
Certain gut microbes promote excessive Th17 activation:
Adherent-Invasive E. coli (AIEC): can hyper-activate Th17 immunity in IBD via IL-23 and CD4 T cells (Munoz et al., 2023)
Eggerthella lenta: Produces a unique enzyme, cardiac glycoside reductase (Cgr2), that can interact with host immunity. E. lenta’s Cgr2 enzyme was found to directly activate Th17 cells (Mehta et al., 2022)
A study by Berer et al. (2017) found that gut microbiota from multiple sclerosis patients can activate Th17 cells and exacerbate disease symptoms when transferred into germ-free mice. This highlights the direct impact of gut bacteria on autoimmune neuroinflammation.
Clinical Pearl:
✔️ Measure microbiome for Th17-promoting bacteria in patients with Th17-driven autoimmunity (MS, psoriasis, Crohn’s disease).
✔️ Implement dietary interventions rich in omega-3s, polyphenols, vitamin D and prebiotics to modulate Th17 responses.
“Some probiotics, like Lactobacillus reuteri, can actually suppress Th17 cells and reduce autoimmunity-related inflammation (Liu et al., 2016)! Chronic low-grade inflammation underpins the development and progression of autoimmune diseases. This persistent inflammatory state results from the interplay of dysbiosis, increased permeability, and immune dysregulation.”
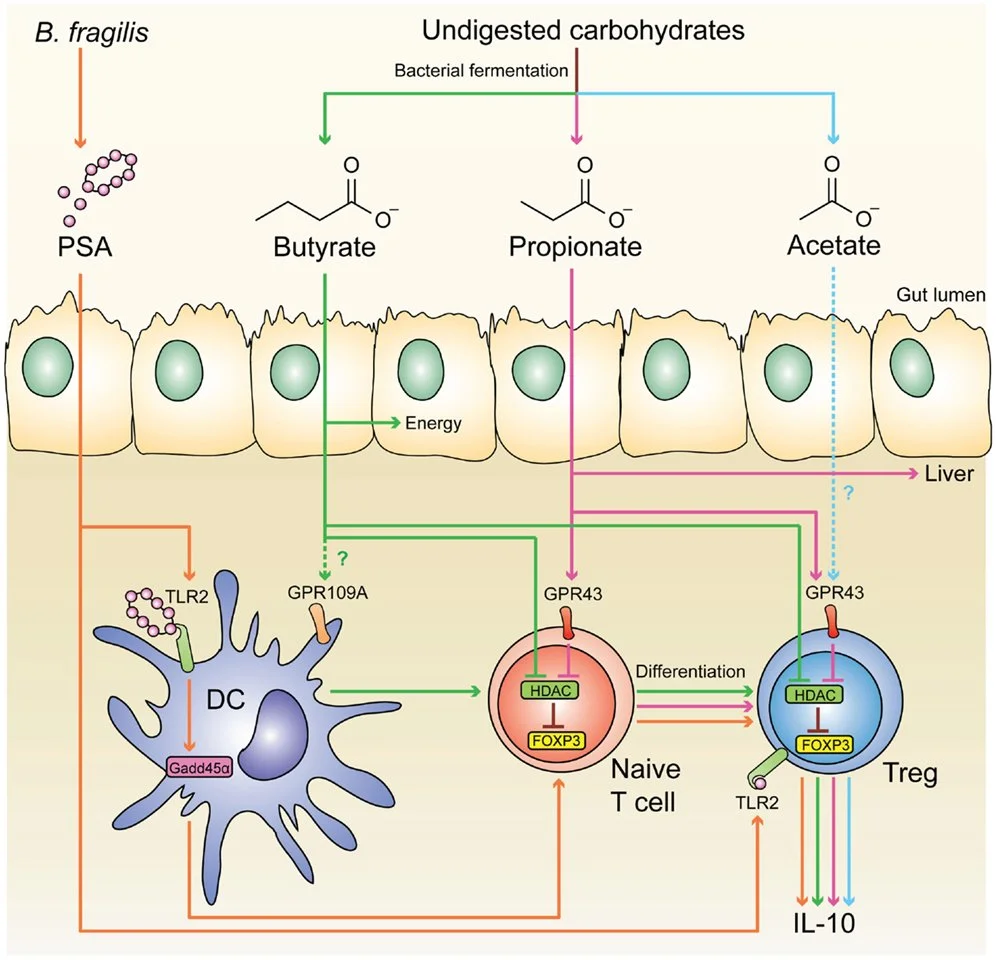
4. Loss of Tregs and Autoimmunity
Regulatory T cells (Tregs) are essential for maintaining immune homeostasis by suppressing excessive inflammatory responses and preventing autoimmunity. A disrupted microbiome can significantly impair Treg function, tipping the balance toward uncontrolled immune activation and tissue damage (Belkaid & Hand, 2014).
Short-chain fatty acid (SCFA)-producing bacteria, such as Clostridia and Bifidobacteria, play a crucial role in inducing Treg development as butyrate directly enhanced the polarisation of Treg cells but not Th17 cells (Smith et al., 2013). Patients with autoimmune diseases often exhibit lower levels of these beneficial bacteria, correlating with diminished Treg populations (Furusawa et al., 2013).
Patients with rheumatoid arthritis and ankylosing spondylitis have significantly lower fecal SCFA concentrations compared to healthy individuals.
Clinical Pearl:
✔️ Support Tregs with SCFA-rich diets (fibre, resistant starch) and probiotics like Bifidobacterium longum.
✔️ Identify dysbiosis-related Treg deficiency in autoimmunity by evaluating SCFA-producing bacteria via stool testing.
Leveraging Functional Testing to Guide Interventions
Functional testing offers valuable insights into the root causes and drivers of autoimmunity, enabling practitioners to design precise interventions.
Key Tests and Their Applications:
Microbiome Testing:
Assess microbial diversity, dysbiosis markers, and SCFA production to tailor prebiotic and probiotic strategies.
Identify overgrowth of pathobionts (e.g., Bilophila wadsworthia) linked to inflammation.
Intestinal Permeability Assessment:
Measure stool zonulin and Hexa-LPS
Elevated markers guide the use of interventions like glutamine, butyrate, and zinc carnosine to restore barrier integrity.
Blood pathology:
Evaluate levels of vitamin D, inflammation, and other key nutrients(e.g., selenium, zinc, B vitamins). Correcting deficiencies directly influences immune regulation and inflammation.
I've hand selected the most important blood markers in Dr Brad Leech: Optimal Health Check
Clinical Tip: Combine testing results with clinical history and symptoms to avoid over-reliance on biomarkers alone.
Managing Your Autoimmune Disease: A Patients Guide to Wellness
About the presentation:
In this 45min presentation, Dr Brad Leech will discuss the intricate world of autoimmune conditions, delving into their root causes and modifiable risk factors. He will shed light on the connections between leaky gut, dysbiosis, inflammation, and immune dysregulation, offering insights into measuring these factors. Dr Brad Leech will present holistic and integrative strategies using diet, lifestyle, and supplements to address these issues and improve your autoimmune health.
Clinically Relevant Conclusion for Practice
Emerging evidence highlights the microbiome’s critical role in autoimmune disease pathogenesis, offering actionable insights for clinical practice. Given the strong link between dysbiosis, intestinal barrier dysfunction, and immune dysregulation, practitioners should incorporate microbiome assessment and targeted interventions into autoimmune disease management.
Key Clinical Considerations:
Microbiome Assessment: Stool analysis (e.g., pathogens, SCFA levels, microbial diversity) can provide valuable insights into gut health and guide interventions. Consider testing in patients with autoimmune conditions, especially those with gastrointestinal symptoms or systemic inflammation.
Restoring Gut Barrier Integrity: Addressing intestinal permeability is essential. Strategies such as increasing dietary fibre intake, supplementing with butyrate-producing prebiotics (e.g., PHGG, RS and beta-glucans), and considering zonulin modulation in high-risk individuals may help reduce microbial translocation and systemic immune activation.
Modulating Immune Responses via the Microbiome:
For Th17-driven autoimmunity (e.g., psoriasis, MS, RA): Reduce inflammatory microbial taxa (Akkermansia, Prevotella) while promoting SCFA-producing bacteria through diet and probiotics.
For loss of regulatory immunity (e.g., lupus, IBD, T1D): Enhance Treg function and IL-10 production via prebiotics and probiotics.
For post-infectious autoimmunity: Consider oral and gut microbiome modulation in patients with a history of infections that may have triggered autoimmunity (e.g., P. gingivalis in RA, Klebsiella in AS).
Targeted Dietary and Lifestyle Interventions:
Increase polyphenol- and fibre-rich foods to support a diverse microbiome.
Reduce processed foods and high-salt diets, which can exacerbate Th17 responses.
Encourage fermented foods and prebiotics for beneficial microbial balance.
By integrating microbiome-targeted strategies into autoimmune disease management, clinicians can address underlying drivers rather than just symptom suppression. A personalised, microbiome-focused approach has the potential to improve patient outcomes, reduce inflammation, and promote long-term immune tolerance.
Dr Brad Leech
Brad is a PhD-qualified Clinical Nutritionist and Herbalist specialising in chronic autoimmune conditions and complex gastrointestinal disorders. He provides complete and personalised care to his patients using functional nutrition, integrative medicine and holistic wellness.